Britain and the vaccine’s developers had until now resisted any restrictions in its use, saying that there was no proof of any link.
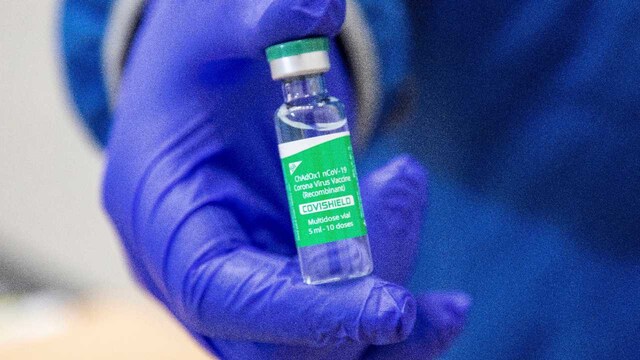
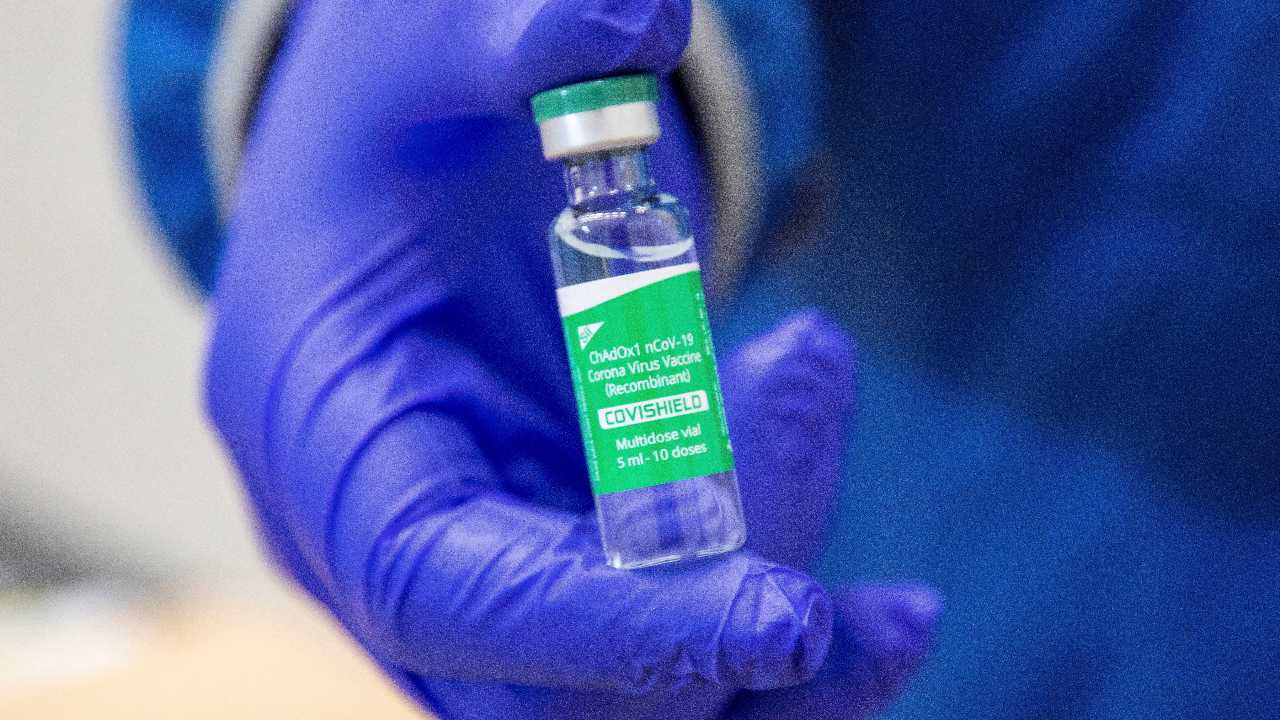
A vial of the AstraZeneca COVID-19 vaccine doses that many nations have secured through a deal with the Serum Institute of India in partnership with Verity Pharma, Wednesday, 3 March 2021, at a facility in Milton, Ontario. Image credit: Carlos Osorio/Pool Photo via AP
A British trial of the AstraZeneca coronavirus vaccine on children has been paused, Oxford University said Tuesday, as global regulators rush to assess its possible link to rare blood clots in adults. The university, which helped develop the embattled vaccine, said in a statement that there were “no safety concerns” in the trial, but acknowledged fears over a potential link to clots by saying that it was awaiting additional data from Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) before restarting the study.
vaccine on children has been paused, Oxford University said Tuesday, as global regulators rush to assess its possible link to rare blood clots in adults. The university, which helped develop the embattled vaccine, said in a statement that there were “no safety concerns” in the trial, but acknowledged fears over a potential link to clots by saying that it was awaiting additional data from Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) before restarting the study.
“Parents and children should continue to attend all scheduled visits and can contact the trial sites if they have any questions,” it added.
It is the latest drama to hit AstraZeneca, which has been embroiled in controversy over its failure to deliver promised doses to the European Union, and over the jab’s efficacy and safety profile.
The MHRA is one of many bodies across the globe analysing real-world data from the AstraZeneca rollout to see if there is a definitive link between the jab and a rare form of blood clot, after cases were initially reported in Norway and continental Europe.
The MHRA reported over the weekend that there had been 30 blood clotting cases, seven fatal, out of the 18 million doses administered in Britain.
The European Medicines Agency said Tuesday it “has not yet reached a conclusion and the review is currently ongoing”.
EU Health Commissioner Stella Kyriakides later said that the agency was expected to make its decision “late Wednesday”, adding that she was in “close contact” with the EMA.
The statement came after the EMA’s head of vaccine strategy Marco Cavaleri was quoted in Italian media as saying that there was a “clear” connection and that the agency would announce it within hours.
“In my opinion, we can say it now, it is clear there is a link with the vaccine,” Cavaleri told Italy’s Il Messaggero newspaper in an interview. “But we still do not know what causes this reaction.”
Germany and France have both restricted use of the vaccine to older people over fears that younger recipients are potentially more at risk from clots.
Britain and the vaccine’s developers had until now resisted any restrictions in its use, saying that there was no proof of any link.
Adam Finn, professor of paediatrics at Britain’s University of Bristol, said that the benefits continued to outweigh the risks.
“We need to know more about the people affected and we need to understand exactly how the illnesses came about,” he said.
“If you are currently being offered a dose of Oxford-AstraZeneca vaccine, your chances of remaining alive and well will go up if you take the vaccine and will go down if you don’t,” he added.