One in 10 Indians will develop cancer during their lifetime, and one in 15 will die of the disease, according to a World Health Organization (WHO) report. The report further stated that all the six most common cancer types – breast cancer, oral cancer, cervical cancer, lung cancer, stomach cancer, and colorectal cancer – combined accounted for 49 percent of all-new cancer cases in the country.
While the information makes us sit up and take notice, it also sheds light on the roadblocks in cancer treatment. For one, all publicly available studies are heavily skewed toward Caucasian data, and there isn’t a good representation of Indian patients. Another challenge is that most large companies have neglected women’s health and there just isn’t enough research on breast cancer, ovarian cancer, or cervical cancers.
Dr. Shibichakravarthy Kannan, Founder and CEO, Oncophenomics, had noticed these unmet needs rather closely. After doing his PhD at the University of North Dakota and a postdoctoral fellowship for three years in lymphoma and myeloma, followed by one year in bioinformatics and computational biology departments at MD Anderson Cancer Center in the US, he came back to India in 2012 to work in the field of oncology and make a difference.
His mission to increase awareness about cancer genomics to promote precision medicine in oncology led him to launch Oncophenomics, a personalized medicine startup. Supported by IKP, BIRAC, AIC-CCMB, and C-CAMP, they developed their prototype and have been selected as one of the winners in the oncology track of the first edition of the Pfizer INDovation Program. The program will help them complete their clinical trials, regulatory milestones and to launch their product.

Identifying the gaps
When we talk about cancer, it is important to understand that this disease is driven by two types of mutations – germline and somatic. Germline is what individuals inherit from their parents, and somatic is acquired through the environment, such as exposure to radiation, toxic chemicals, or other DNA-damaging factors. Hence, it is a combination of genetics and the environment.
“Cancer patients keep asking me, ‘there are billions of people in the world. Why did I get cancer? Why me?’ The answer is very simple. It’s a simple statistical probability. DNA gets damaged by various sources. In normal individuals, the DNA repair machinery works very well. Also, the immune system keeps cancer at bay. However, in cancer patients, the DNA repair machinery itself is at fault. The mutations keep accumulating, and the immune system fails to eliminate cancer. This is what we call the multiple hits hypothesis. So, someone with a germline hit, has a genetic predisposition to develop cancer in their lifetime, but cancer doesn’t start until there are multiple hits or triggers from the environment, a cumulative effect of more and more mutations acquired over several years leading to carcinogenesis. This fundamental understanding of cancer biology is lacking in the current ecosystem. We need to create awareness for cancer screening programs. Early detection is the key to controlling this disease. Today we have several new targeted therapies and immunotherapies which give better results when treated early,” Dr. Kannan explains.
“In an ideal scenario, every cancer patient should get a DNA test done. But that is not happening. Less than 5 percent of patients opt in for a genomic test. Whenever professionals are looking at new treatment options, especially in breast and ovarian cancer, they want to see the BRCA Panel. Based on this, they can recommend new drugs such as PARP inhibitors, but these scenarios arise only if they do the genomic test. For a majority of breast and ovarian cancer patients, surgery followed by chemotherapy remains the standard of care, with less than 40% success rates and a high prevalence of cancer recurrence or metastasis within a year. We need a paradigm shift in the cancer treatment landscape – we need to promote personalized medicine instead of the one-size-fits-all chemotherapy,” he adds.
Oncophenomics is on a mission to create awareness about both the need for genomic testing as well as the newer options for treatment. Since genomics has advanced in the past 20 years, pharma companies have developed over 300 drugs in this new category called targeted therapies and almost 30 new drugs under immunotherapy. Apart from the three standard-of-care treatments, targeted therapies and immunotherapies have come up as the fourth and fifth umbrella of treatment modalities.

Unfortunately, patients are still treated with standard-of-care therapies in all public hospitals. “The doctors don’t even think about targeted therapies or immunotherapies because the cost is a major concern, and doctors don’t know the efficacy of the many new drugs in the market’,” Dr. Kannan further adds.
Oncophenomics aims to counter these challenges with a three-pronged approach – by bringing the latest technology to India, making it accessible and affordable for everyone, and making the comprehensive genomic profiling liquid biopsy test results easy to understand, so that it can yield clinically actionable results.
Leveraging liquid biopsy for early cancer detection
Dr. Kannan’s work with liquid biopsy testing started five years ago, when he was working with a diagnostic lab called Datar Cancer Genetics. There, he was part of the team that developed a liquid biopsy test. Since its development, the test has gone through several iterations over the last couple years, and recently received Breakthrough Designation from the US FDA. Inspired, Dr. Kannan focused on developing a liquid biopsy test for India, since no such offerings were available here at the time. He engaged with several oncologists to validate his idea and need for a liquid biopsy test.

About these interactions, Dr. Kannan said “It’s a non-invasive test and several companies are already doing it in the US. The doctors wanted to know if this technology was available in India, and if so, then how soon could their patients get access to it. So, we reached out to several large foreign companies, but none of them were ready to come to India as most of them were offering it as a lab-developed-test under CLIA guidelines. The blood samples had to be shipped to US, EU or Singapore, and the tests were unaffordable to most patients in India. This motivated us to develop a 100% Make-In-India liquid biopsy test that addresses the unique population genetics of the Indian population”.
He launched Theranosis Life Sciences Pvt Ltd in 2016, which was later rebranded as Oncophenomics in April 2019. While there are several use cases for liquid biopsy, the most promising is the clinical utility as a Companion Diagnostics test. Leveraging the latest, third generation NGS technologies, Oncophenomics’ liquid biopsy test will help oncologists to personalize the treatment plan for each cancer patient. Oncophenomics mission is to detect cancer recurrence, relapse, or metastasis several weeks earlier than the conventionally used imaging modalities. By mapping the tumor mutational landscape, they can predict which targeted therapies or immunotherapies will work for the patient. Currently, the team is focused on getting their liquid biopsy test market-ready for women’s cancers like breast, ovarian, endometrial, and cervical cancers.
“With several big players already addressing melanoma, brain, prostate, lung, and colorectal cancer, we had to choose a niche market with unmet needs, where there’s not much competition. But at the same time, we also wanted to create strong social impact. Early diagnosis for both primary and secondary cancers have been proven to improve survival and quality of life, and women’s cancers have high incidence and typically get diagnosed very late. Thus, we began with a focus on these cancers,” Dr Kannan reveals.
Making genomic testing accessible
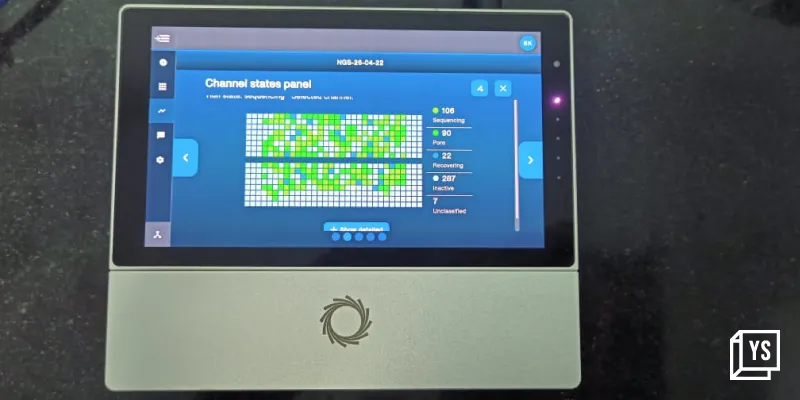
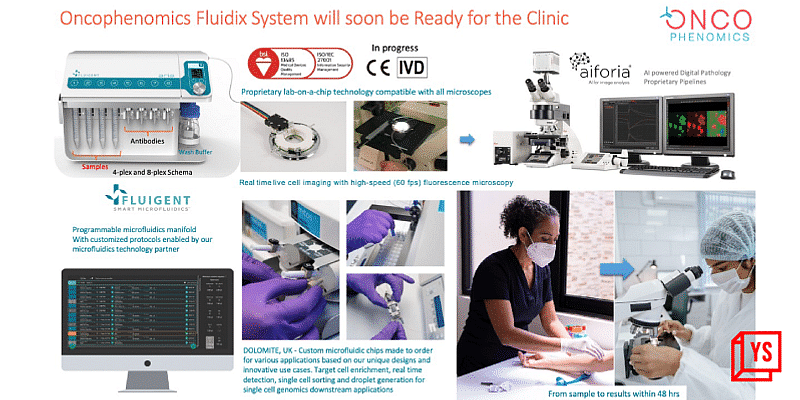
Oncophenomics has developed three types of liquid biopsy – CTCs, ctDNA and Exosomes. The first one is an innovative microfluidics lab-on-a-chip technology for the detection and capture of viable circulating tumor cells (CTCs) from a simple blood sample. Being non-invasive, it can be repeated as many times as required for real-time treatment monitoring and evaluating clinical trial outcomes. This microfluidics platform is currently a Research-Use-Only (RUO) test for cancer research, drug discovery and development use cases. The second type of liquid biopsy is a cell-free DNA (cfDNA) based test, that is also offered by a few other companies in India and abroad. Unlike tissue biopsy, there is no sampling bias in cfDNA liquid biopsy. It gives doctors a comprehensive picture of the patient and the entire body in a holistic way, unlike imaging techniques, including CT, MRI or PET-CT, which capture one or two lesions. “The 3rd generation NGS technology that we have adopted, Nanopore sequencing, has a very high sensitivity and specificity for detecting very rare cfDNA fragments of tumor origin (ctDNA). The limit of detection (LOD) is million times better than older short read technologies like Illumina or Ion Torrent which are currently in use. Oncophenomics can detect a few ctDNA among billions of normal cfDNA fragments – it’s like finding the needle in the haystack,” Dr. Kannan explains.
Coming to accessibility, Dr. Kannan wants to create a decentralized hub-and-spoke business model where they will go to every nook and corner, every clinic, every cancer hospital, or a public hospital, and deploy their platform in their facilities. “Almost every hospital has an in-house pathology lab. We want to deploy there while providing them with the necessary training required to process the samples. Once the sequencing run starts, the data will stream to our server in real-time. Our fully automated bioinformatics pipelines will analyze the raw data and generate a draft report which will then be evaluated by a qualified panel of scientists and pathologists. Aided by artificial Intelligence, this panel will enable same-day reporting compared to the current 3 to 4 weeks waiting time for a DNA test report.” he says.
Talking about affordability, Dr. Kannan says, “Instead of a Rs.3,75,000 test, we wanted to develop a Rs 37,500 test, which is 1/10th the cost. That was our initial pricing. Now we are offering the test at Rs 25,000, which is $300. Eventually, once we scale up the operations, we can offer the test at $99 per patient. Our agenda here is to build the Indian cancer genome reference database. We don’t want to make profitability on the per-test cost if we can cover the cost of flow cells and reagents. We plan to democratize access to this database to further fuel cancer research in India.”
He notes that most of the international studies are based on Caucasian women and selected ethnicities like the Ashkenazi Jewish population. “When we test the same primers and probes in India, it comes negative most of the times. Most BRCA tests done by diagnostic companies in India tend to provide false negative reports, because those specific mutations are not found in our population. CSIR-IGIB has recently published BRCA mutations unique to Indian women. Oncophenomics is working on building a comprehensive cancer patient database for the Indian population.
Scaling with Pfizer INDovation Program
As one of the winners at Pfizer INDovation under the oncology track, Oncophenomics will receive a grant of Rs 65 lakh. Speaking about the program, Dr. Kannan says that they applied to get industry guidance, mentorship, visibility, and connections from the Pfizer and Social Alpha team.
“There are two main business use cases for the liquid biopsy test that we have developed. One is the clinical use case for patients on a one-on-one basis. And the bigger use case is working with pharma companies. Pfizer was the perfect partner to validate our offerings. The Social Alpha team has also been very supportive and helpful in creating a robust Go-To-Market strategy,” he explains.

Oncophenomics is focusing on upgrading its facilities and working on setting up IVD Kit manufacturing processes. In the next two years, the team is looking at a presence in at least 10 locations in the country, starting with private hospitals like Apollo Hospitals as well as several public cancer hospitals
“We will give them the hardware, the equipment, the kits, and the reagents. It’s like a complete NGS facility, where we upgrade a regular pathology lab into a full-fledged clinical genomics lab so that they can start processing samples daily. They can process at least 12 to 24 samples every day. If they have the budget, they can opt for bigger instruments and process between 96 to 2048 samples every day. We are looking at a digital transformation of pathology labs and we will completely handhold them in this process. We will focus more on data analytics and building predictive data analytics. We will become the data custodians while protecting patient privacy as per national and international guidelines. Eventually, once we cross 10,000 patient samples, we can bring in artificial intelligence tools to extract meaningful insights from this massive data warehouse,” Dr. Kannan shares his long-term vision.





![Read more about the article [Funding alert] Dailyhunt parent VerSe Innovation raises $450M in Series I round](https://blog.digitalsevaa.com/wp-content/uploads/2021/08/Umang-Bedi-Viru1561025189604-1614064889755-300x150.png)