Dialysis is a life-sustaining treatment for patients with End-Stage Renal Disease (ESRD). Globally, the dialysis population is growing, particularly in low-income and middle-income countries.
“A substantial number of people lack access to kidney replacement therapy, and millions of people die of kidney failure each year, often without supportive care,” says a report.
Here are the ten top international startups shortlisted for the 2021 Future Hamburg Award
The cost of dialysis care remains high, and patients on dialysis continue to endure various difficulties including, low health-related quality of life, the burden of disease, and shortened life expectancy.
Despite the expansion in the provision of dialysis, the rate of true patient-centred innovation has slowed down in the recent past, especially on hemodialysis.
As a result, there is a necessity to develop new approaches and dialysis modalities that are cost-effective, flexible, accessible, and improve patient outcomes.
Quanta Dialysis Technologies’s mission
Here’s where Quanta Dialysis Technologies (Quanta) becomes relevant. Based out of Alcester, UK, Quanta is a medical devices company that develops advanced haemodialysis systems for homes and clinics.
Just so you know, Hemodialysis is a life-sustaining treatment for patients with kidney failure to help normalise blood chemistry and remove waste products and excess fluids.
The UK company is on a mission to improve the delivery of dialysis and help people live more freely.
Raises €205M
Recently, Quanta has secured $245M (approx €205M) in an oversubscribed and upsized Series D round. Notably, this is the largest private funding round for a dialysis device company in history.
Who backed Quanta?
The round was led by Glenview Capital and co-led by Novo Holdings, with support from a broad group of other top-tier investors, including BlackRock, Eldridge, Sands Capital, Millennium Management, Monashee Investment Management LLC., Puhua Capital, Segulah Medical, and Ancora, alongside Orlando Health, an integrated delivery network. Existing shareholders also participated in the round, including Wellington Partners, btov, Seroba Life Sciences, and The Grands.
Lee Hathaway, Partner and Co-Head of Healthcare at Glenview Capital, and Robert Ghenchev, Senior Partner at Novo Holdings and Head of Novo Growth, will be joining the Quanta Board of Directors.
Fund utilisation
The funding will be used to scale up global operations with a focus on the $12B (approx €10B) US market. Earlier this year, the company received 510(k) clearance from the US Food and Drug Administration (“FDA”) to market its hemodialysis system SC+.
The company is also planning to scale up manufacturing, sales, and customer service functions to support use in currently approved care settings within the US. On the other hand, it is preparing to launch a study to support its future FDA clearance for in-home use within the US.
“Innovation in kidney dialysis has been stagnant for too long – but the demand from patients, doctors, health care providers, and payors for new, better, and more flexible solutions is impossible to ignore,” says Johan de Ruiter, Chairman of Quanta. “We are excited to scale up our operations, enabling us to provide access to this critical new technology that will save lives, improve patients’ quality of life, and lower healthcare costs.”
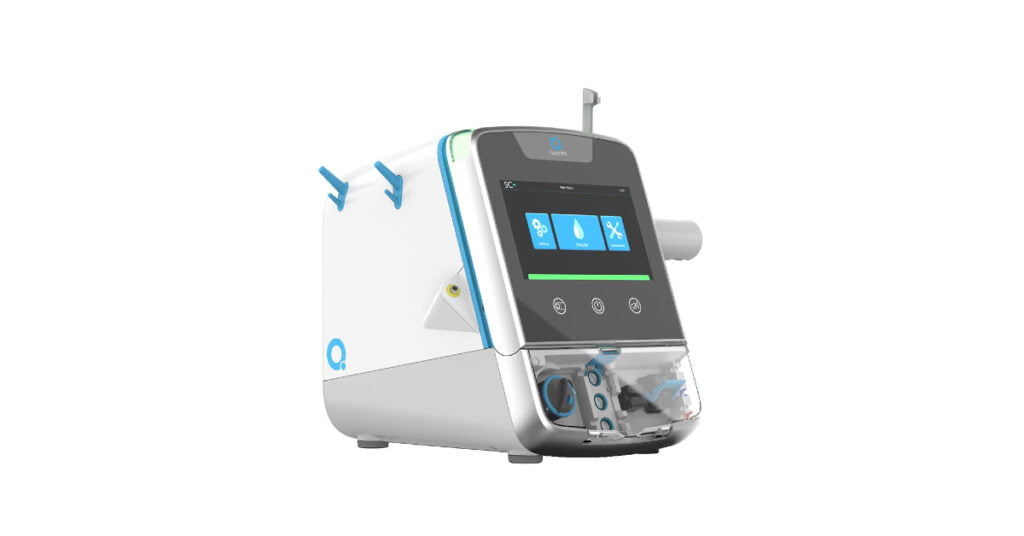
Compact and flexible haemodialysis system
Quanta’s SC+ is a compact haemodialysis system providing clinical efficacy by utilising typical flow rates of traditional in-centre machines. The lightweight design and compact form factor allow the dialysis treatments to be brought to patients.
“For vulnerable patients in hospital and post-acute settings, being able to access dialysis on-site can mean better outcomes and lower costs of care,” says the company in the press release.
To complement SC+, Quanta consists of a suite of related products and services. It includes an optional, portable water purification module that will enable SC+ to be easily moved around and to be operated in a wide range of settings, both with and without a centralised water system.
Secondly, cloud-based digital health offering that will simplify and automate treatment data capture and reporting, negating the need to create and store manual records.
Treatments in the US and UK
In the US, SC+ is FDA cleared for use in acute and chronic care facilities, and in the UK, it is CE marked, where it has been successfully used to treat patients across a range of care settings, from the ICU and the clinic to the home.
John E. Milad, CEO of Quanta, says, “Everybody knows that dialysis care must improve. For this to happen, providers and physicians need products that allow greater flexibility to bring dialysis directly to the patient while simplifying complexity and reducing the overall cost of care. Our small, simple-to-use, and powerful dialysis system SC+ is positioned to help transform kidney care. The funding we are announcing today will allow us to accelerate our emergence as a significant force in this market.”
Back in February, Quanta appointed Dr Selwayan Saini as Chief Operating Officer to drive product development and other aspects of the company.
Here’s how to build one that doesn’t, according to this expert…





![Read more about the article [Funding Galore] Over $1.4 Bn Raised By Indian Startups This Week](https://blog.digitalsevaa.com/wp-content/uploads/2021/12/Social_Image-dec-20-dec-25-300x157.jpg)



