India’s COVID-19 situation is worsening every day. According to reports, the country has about 15.6 million cases as of now recording an average daily surge of over two to three lakh infections.
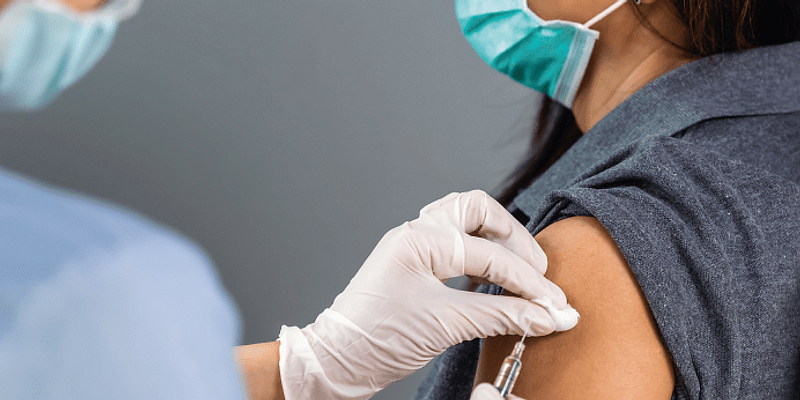
In a bid to fight against the infectious disease, the central government has opened up vaccination against COVID-19 for all those who are 18 years and above from May 1.
On Monday, the central government also revealed a new vaccination policy which stated that vaccine manufacturers will need to supply 50 percent of their monthly Central Drugs Laboratory (CDL) released doses to Government of India and will be able to supply the remaining 50 percent doses to state government and in the open market.

Prime Minister Narendra Modi
“The government has been working hard from over a year to ensure that maximum numbers of Indians are able to get the vaccine in the shortest possible of time,” Prime Minister Narendra Modi said.
With offices, local transportation, markets, malls running full-fledged operations, it is indeed important for the working population to get vaccinated and take precautions.
With a large part of the population taking vaccines, manufacturers are working overtime to ramp up their production.
Ramping up production
In order to meet the upcoming demand, the central government has approved payment of about Rs 4,500 crore as advance to Serum Institute of India (SII) and Bharat Biotech.
Welcoming the announcement on expanding the vaccine drive, SII has stated that it will be working to address the issue of limited capacity by scaling up the vaccine production.
Complying with the new rule of providing 50 percent doses to state government and open market, Covishield vaccine will now be available at Rs 400 for state governments and Rs 600 for private hospitals.
“Owing to the complexity, and urgency of the situation it is challenging to supply it independently to each corporate entity. We would urge all corporate and private individuals to access the vaccines through the state facilitated machinery and private health systems. Post four to five months, the vaccines will be made available in retail and free trade,” SII said in a statement.
According to government data, Covishield comprises over 90 percent of the 12.76 crore COVID-19 vaccines administered across the country
Apart from this, Covaxin maker Bharat Biotech also said it will produce 30 million doses of its COVID-19 vaccine. The company stated that it has ramped up its production capacity of Covaxin to 700 million doses per annum.
“Last month, we produced 15 million doses. This month, we are reaching 20 million doses; next month, we will be making around 30 million doses followed by 70 to 75 million doses. We are ramping up the production and by July-August, we will be able to reach 700 to 800 million doses production capacity per annum,” said Krishna Ella, Chairman and MD at Bharat Biotech.
On Wednesday, the company revealed that Covaxin has shown the efficacy of 78 percent against mild, moderate, and severe cases of COVID-19 as per Phase III interim analysis results. It also added that results from the final analysis will be available in June.
New vaccines in India
In order to meet the increasing demand for vaccines, India is also looking at introducing new vaccines to accelerate the fight against COVID-19.
Earlier this month, Russian COVID-19 vaccine Sputnik V got permission for restricted emergency use with certain conditions from the Drugs Controller General of India (DCGI)
With this approval, Sputnik V has become the third approved vaccine in India against coronavirus. Hyderabad-based pharmaceutical company, Dr. Reddy’s Laboratories, which was conducting the Indian trials for Sputnik V will be importing the vaccine for emergency use in India.
According to the PTI report, the Sputnik V vaccine is expected to arrive this month or latest by next month.
Apart from this, Moderna, and Johnson and Johnson have also been urged to seek emergency use approval in India. In order to meet the vaccine demand, the government is also reportedly considering waiving off 10 percent customs duty levied on imported vaccines to lower the costs of overseas vaccines
While vaccine makers and the government is doing their bit to ramp up vaccination efforts to eliminate coronavirus, we can only defeat the pandemic when we follow the advised protocols and take appropriate precautions. Vaccination is a preventive measure and not a cure. With the second wave of infections hitting the country, it is the need of the hour to take measures and cut the curve to eliminate the disease.
[With inputs from PTI]



![Read more about the article [Funding Alert] Orange Health raises $25M from Bertelsmann India Investments, others](https://blog.digitalsevaa.com/wp-content/uploads/2022/06/Imagefl6t-1654064962080-300x150.jpg)


![Read more about the article [Startup Bharat] How Coimbatore-based Machenn Innovations is upskilling engineering students to acquire industry-level skills](https://blog.digitalsevaa.com/wp-content/uploads/2021/06/imageonline-co-logoadded45-1624534956731-300x150.jpg)


