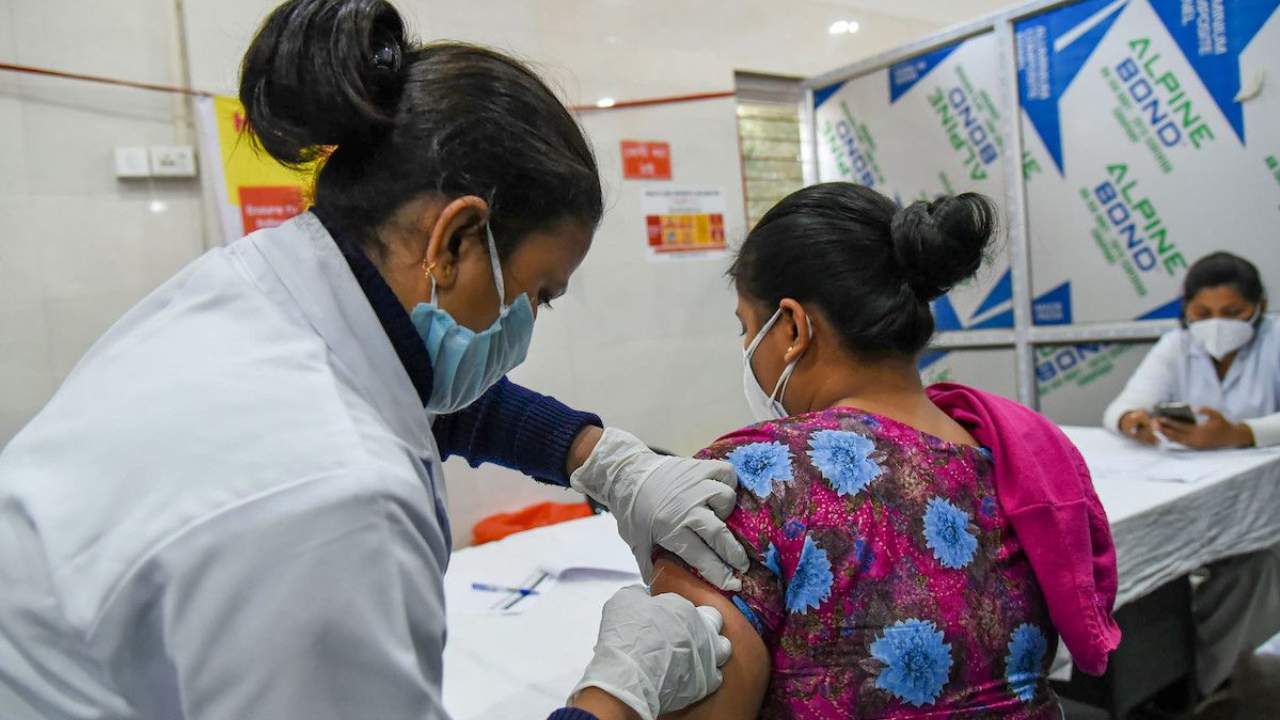
If granted, the request would loosen the stringent conditions under which the vaccine is being administered, and possibly improve public acceptance of the vaccine.
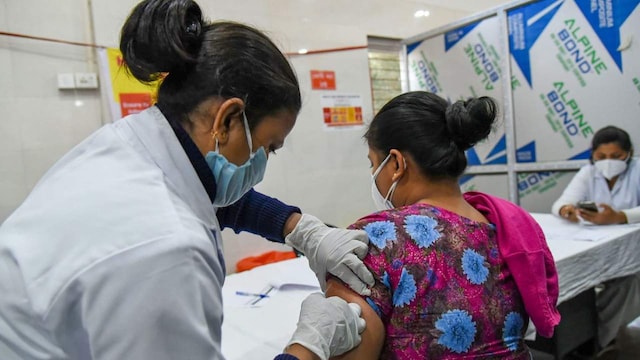
Two months ago, the vaccine’s emergency use authorization was permitted only in the clinical trial mode. The Covid-19 subject expert committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO) on Wednesday approved Bharat Biotech’s Covaxin for emergency use authorisation in India. Image: PTI
A panel of experts has recommended the removal of the “clinical trial mode” label attached to the emergency authorisation given to Bharat Biotech for its COVID-19 vaccine Covaxin. The recommendation by the Subject Expert Committee (SEC), if accepted by India’s top drug regulator, would loosen the stringent conditions under which the vaccine is currently being administered, and might lead to better acceptance of the vaccine. The SEC, on reviewing a request that Bharat Biotech had put in earlier this week, has recommended that the regulator grant it an approval “like Covishield”, according to a report in the Indian Express.
vaccine Covaxin. The recommendation by the Subject Expert Committee (SEC), if accepted by India’s top drug regulator, would loosen the stringent conditions under which the vaccine is currently being administered, and might lead to better acceptance of the vaccine. The SEC, on reviewing a request that Bharat Biotech had put in earlier this week, has recommended that the regulator grant it an approval “like Covishield”, according to a report in the Indian Express.
Now, the vaccine maker is awaiting a review of the SEC’s recommendation to the Drugs Controller General of India (DCGI) VG Somani, to remove Covaxin from the ‘clinical trial mode’ condition it is currently tied to.
Covaxin is India’s first indigenous COVID-19 vaccine, being developed and trialled by Hyderabad-based Bharat Biotech. Based on a Phase 2 study and 3-month follow-up, Bharat Biotech reported that Covaxin was almost 81 percent effective in preventing COVID-19
vaccine, being developed and trialled by Hyderabad-based Bharat Biotech. Based on a Phase 2 study and 3-month follow-up, Bharat Biotech reported that Covaxin was almost 81 percent effective in preventing COVID-19 disease, ‘safe’ and ‘immunogenic’, in a Lancet study published 3 March 2021. The scientific and medical communities are yet to look at a thorough evaluation of the vaccine’s efficiency – specifically: data from the phase 3 human trials, in which the vaccine’s effectiveness in preventing COVID-19
disease, ‘safe’ and ‘immunogenic’, in a Lancet study published 3 March 2021. The scientific and medical communities are yet to look at a thorough evaluation of the vaccine’s efficiency – specifically: data from the phase 3 human trials, in which the vaccine’s effectiveness in preventing COVID-19 disease is probed in a sample of the population(s) it is intended for.
disease is probed in a sample of the population(s) it is intended for.
The vaccine maker also conceded that safety outcomes were not evaluated in the Phase 2 trial, and “extensive Phase 3 trails” were needed to evaluate the risks and contraindications of Covaxin, as per the study.
“We were unable to assess other immune responses (i.e., binding antibody and cell-mediated responses) in convalescent serum samples due to the low quantity,” the study said. In other words, we can expect to kn

An illustration of COVAXIN, the vaccine candidate for COVID-19 developed by Bharat Biotech. Image: Bharat Biotech
developed by Bharat Biotech. Image: Bharat Biotech
ow more about the immunologic protection offered by the vaccine (antibody response, efficacy against SARS-CoV-2 virus variants, etc) in the subsequent Phase 3 report. Part of the reason these couldn’t be evaluated
The subject expert committee of CDSCO is likely to meet on Thursday, according to a report in Hindustan Times, to review a request from Bharat Biotech to remove the ‘clinical trial mode’ tag currently attached to Covaxin.
So far, the government has required that Covaxin be used under ‘clinical trial mode’, where all measures followed when a volunteer is given the shot during a clinical trial (like informed consent, close and active follow-up of the recipient for a fixed period of time) are followed, with the vaccination.
Covaxin is one of two vaccines being used widely to vaccinate the Indian population in a phased manner via a national vaccination drive.